The Muon Spectroscopy Computational Project
Software and methods to make the muon spectroscopist's life easier

People
Publications
Tutorials
Software
Funding
Contributing
Symmetry analysis method
Symmetry considerations are important for determining the muon stopping sites. In crystallography, the sites with high symmetry are often the preferred atomic sites. This holds true for muons as well, and in practice, very often, the muon stopping site is an intertitial high-symmetry site of the crystal. For this reason, a crystallographic analysis of the pure system that will be studied with muons is a useful first step when looking for muon stopping sites, as such analysis is significantly faster than any computer simulation.
A detailed description of the methodology is given in this paper by S. Sturniolo and L. Liborio.
Software implementation
The symmetry analysis can be found in the Python library we deployed specifically to aid muon computational science: pymuon-suite. The library can be found on Github and is released under a GNU v3.0 open source license.
Once installed, pymuon-suite provides the user both with a Python API to use for custom programs and with a taylored pm-symmetry script, which can be used to perform the symmetry analysis.
The symmetry script can be run simply by executing the command:
pm-symmetry <structure file>
where the structure file has to be any supported crystallographic file format (such as .cif or or the CASTEP structure file .cell).
Example of application of the symmetry method
TiO2 rutile
The muon stopping sites in TiO2 rutile were determined by transverse field μSR measurements performed in the MUSR instrument at ISIS(UK). In the these stopping sites, the muon has a low temperature ground state and a high temperature excited state, both corresponding to a muon bound to one of the six oxygen atoms that form an octahedron around the Ti3+ at the center of the TiO2 rutile unit cell. Each one of these stopping sites has a different O–Ti3+ bonding configuration, with the ground state formed by bonding the muon to the in-plane oxygen atoms that lie in the same plane as Ti3+. These two sites are related by symmetry and are only distinguished by the electronic structure of the TiO2 rutile.
Running pm-symmetry on a CASTEP TiO2 rutile structural file rutile-out.cell produces the following output:
`
Wyckoff points symmetry report for rutile-out.cell
Space Group International Symbol: P4_2/mnm
Space Group Hall Number: 419
Absolute Fractional Hessian constraints
[0. 0. 1.47026712] [0. 0. 0.5] none
[0. 2.33609456 0. ] [0. 0.5 0. ] none
[0. 2.33609456 0.73513356] [0. 0.5 0.25] none
[0. 2.33609456 1.47026712] [0. 0.5 0.5] none
[0. 2.33609456 2.20540069] [0. 0.5 0.75] none
[2.33609456 0. 0. ] [0.5 0. 0. ] none
[2.33609456 0. 0.73513356] [0.5 0. 0.25] none
[2.33609456 0. 1.47026712] [0.5 0. 0.5] none
[2.33609456 0. 2.20540069] [0.5 0. 0.75] none
[2.33609456 2.33609456 0. ] [0.5 0.5 0. ] none
`
The coordinates in bold indicate the muon stopping site that agrees with the experimental results. The predicted stopping site is shown in the figure below. As we mentioned above, the muon is bonded to one of the six oxygen atoms that form an octahedron around the Ti3+ atom, which is shown at the center of the TiO2 rutile unit cell in this figure:
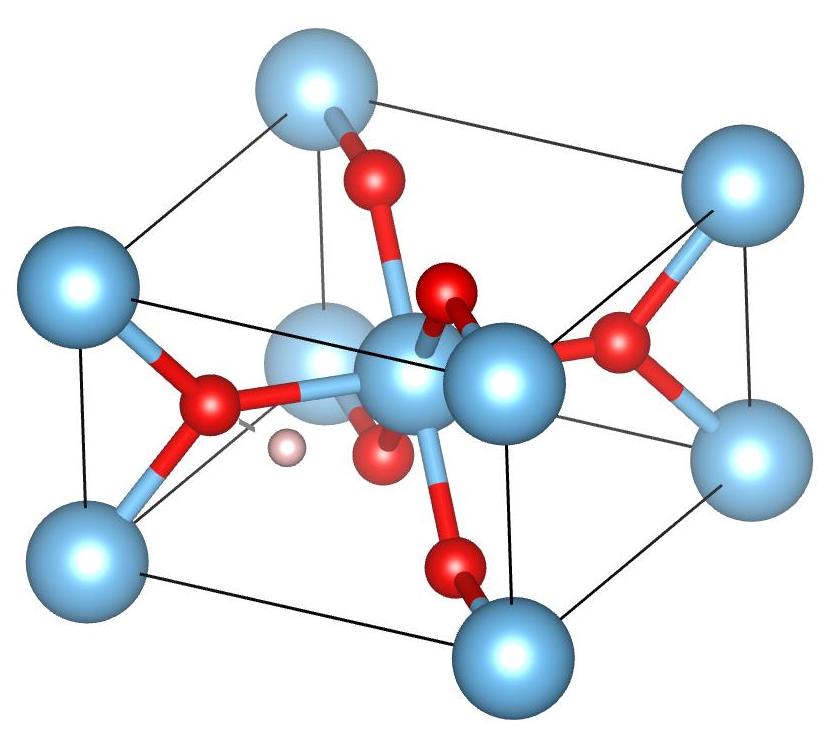
So, it is clear that there is a connection between the symmetry properties of the crystalline material and the potential muon stopping sites in that material. The symmetry method is an extremely fast method. However, if possible, this method should usually be used in conjunction with experimental results and physical intuition. It is the case that there may be a significant number of high symmetry points in a given crystalline material and, unless there is some extra information available, it is difficult to discriminate between potental stopping sites.
Hosted on GitHub Pages — Theme by orderedlist